Determining The Type Of Atom By Inventing A New Method
The Researchers Were Able To Invent A New Method To Identify The Type Of Atom And Its Chemical State Using The Synchrotron X-Ray Scanning Tunneling Microscope ( SX-STM ).
Atomic-scale imaging emerged in the mid-1950s and has been advancing rapidly ever since. So much so that in 2008, physicists successfully used an electron microscope to image a hydrogen atom.
Five years later, scientists could probe inside the hydrogen atom using a quantum microscope, resulting in the first direct observation of electron orbitals. According to a new paper published in Nature, we now have the first X-rays taken from a bit, conducted by scientists at Ohio University, Argonne National Laboratory, and the University of Illinois-Chicago.
“Atoms can be routinely imaged with scanning probe microscopes, but without X-rays, you can’t tell what they’re made of,” says Saw-Wai Hla, a physicist at Ohio University and Argonne National Laboratory.
We can now precisely identify the type of a particular atom, and we can simultaneously measure its chemical state. Once we can do this, we can trace materials down to the size of an atom. This will have a significant impact on environmental science and medicine.
When people think of an atom, they most likely imagine the popular classical version of Bohr’s atomic model. This is where the electrons move in circular orbits around the atom’s nucleus like the planets in our solar system orbit the sun.
Orbits have discrete energies, which are related to a rotation’s size. In this way, the lowest point is associated with the most miniature circuit.
Whenever an electron changes its speed or direction according to Bohr’s model, it emits radiation at specific frequencies associated with particular orbitals.
 In 2013, physicists used a quantum microscope to look inside a hydrogen atom.
In 2013, physicists used a quantum microscope to look inside a hydrogen atom.
This model has changed over time since Niels Bohr proposed it in 1913. This is due to advances in our understanding of the quantum world. Erwin Schrödinger proposed a new atomic model that abandoned orbitals in favor of energy levels.
It still has similar concepts to Bohr’s model. For example, if an atom is heated (i.e., becomes more energetic), its electrons move to higher levels. As they cool and return to their original state, the excess energy has to go somewhere, so it is emitted as photons, and those photons have frequencies that correspond to the change in energy levels.
Technically speaking, electrons don’t move around the nucleus in orbits. Electrons are waves. But when you experiment to determine their position, they appear as particles, and these waves are stationary. You can check where an electron is, but each time you do it, it appears in a different position, and that’s because the states interact, not because it’s moving. Anyway, if you Don’t look at its electron, it doesn’t have a fixed position, and the wave function collapses.
The researchers believe that if you take many individual measurements and plot the electron positions for each one, you’ll have a ghost-like orbital pattern much closer to what an atom looks like.
 When X-rays (blue color) shine on an iron atom (red ball in the center of the molecule), the surface electrons of the nucleus are excited.
When X-rays (blue color) shine on an iron atom (red ball in the center of the molecule), the surface electrons of the nucleus are excited.
X-ray excited electrons are then tunneled to the detector tip (gray) through atomic/molecular orbitals that provide elemental and chemical information about the iron atom.
As Hla points out, physicists can now routinely image atoms with scanning probe microscopes. This method works by placing a very sharp tip on the surface and forming an image of the character from the signal read by the end. Similar to a player that reads the grooves on a screen to play sound. The first of these techniques, scanning tunneling microscopy (STM), was developed by IBM researchers in 1981. STM relies on quantum mechanical tunneling effects. Electrons travel from the tip to the tunnel surface as the microscope tip is reviewed over a surface. The tunneling current is measured and can be visualized.
For the past 12 years, Hla has been developing an X-ray version of the STM called the Synchrotron X-ray Scanning Tunneling Microscope, or SX-STM, which enables scientists to identify the type of atom and its chemical state. X-ray imaging techniques, such as synchrotron radiation, are widely used in countless fields, including art and archaeology.
But the smallest that can be done with X-rays to date is one attogram or about 10,000 atoms.
This is because the X-ray emission from a single atom is so weak that it has not been detected until now.
SX-STM combines conventional synchrotron radiation with quantum tunneling. It replaces the traditional detector of X-ray used in most synchrotron radiation experiments with a different type of detector, which is a sharp metal tip placed very close to the sample to capture better the electrons that have been pushed into an excited state by the X-rays. to collect.
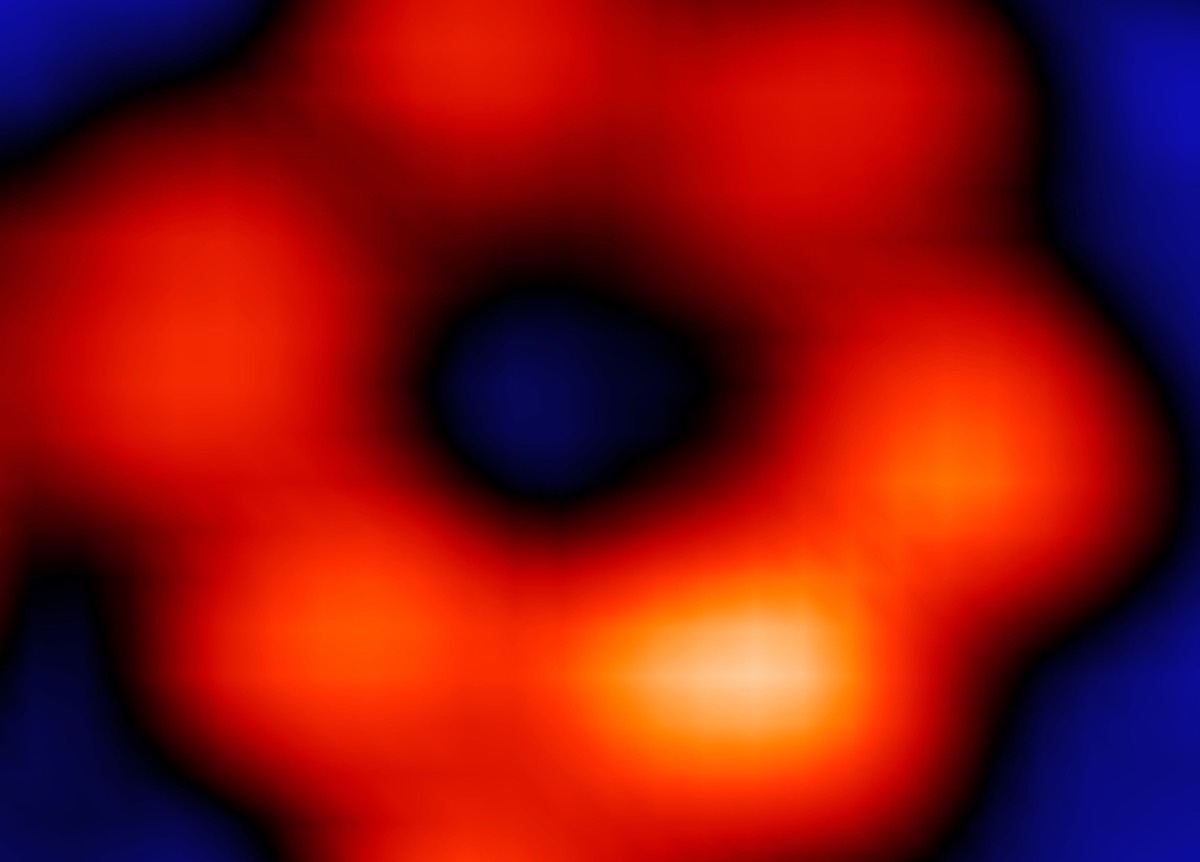
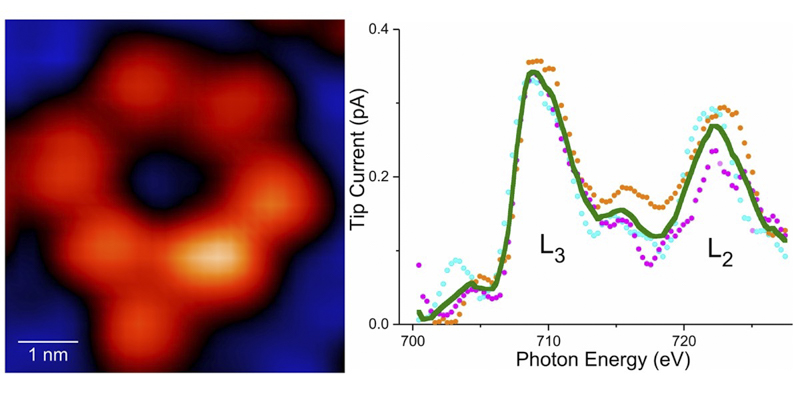 (Left) An image of a ring-shaped supermolecule with only one iron atom in the entire ring. (Right) X-ray effect of a single iron atom.
(Left) An image of a ring-shaped supermolecule with only one iron atom in the entire ring. (Right) X-ray effect of a single iron atom.
Optical absorption of core electrons acts as an elemental fingerprint to identify the type of atoms in a substance. With Hla et al.’s method, X-rays hit the sample and excite the core electrons, tunneling into the detector tip. The team tested their method at the XTIP beamline at the Argonne Advanced Photon Source using an iron atom and a terbium atom.
Emphasizing that this is not the whole story, this physicist said:
We have also identified the chemical states of individual atoms. By comparing the chemical conditions of an iron atom and a terbium atom in their respective molecular hosts, we find that the terbium atom, a relatively isolated rare earth metal, does not change its chemical state.
In contrast, the iron atom strongly interacts with its surroundings. It interacts. Hla’s team also developed another technique called X-ray Excited Resonance Tunneling (X-ERT), which allows them to detect the orbital orientation of a single molecule on the material’s surface.
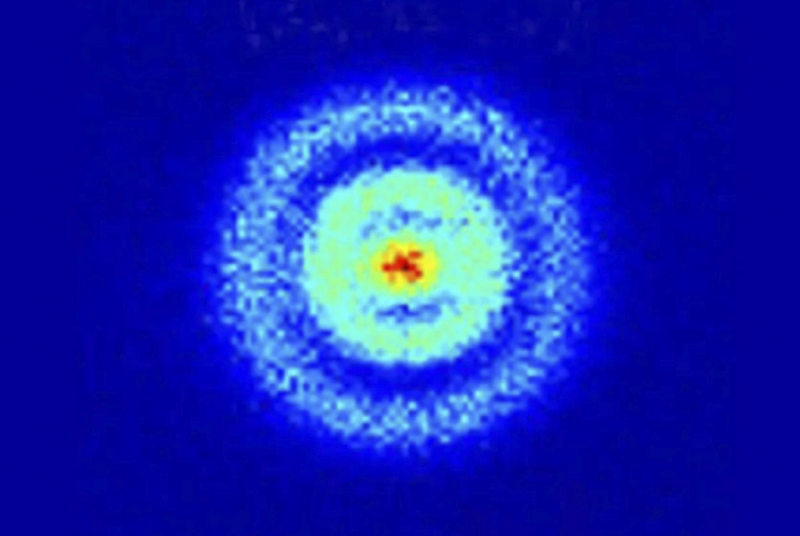 In 2013, physicists used a quantum microscope to look inside a hydrogen atom.
In 2013, physicists used a quantum microscope to look inside a hydrogen atom.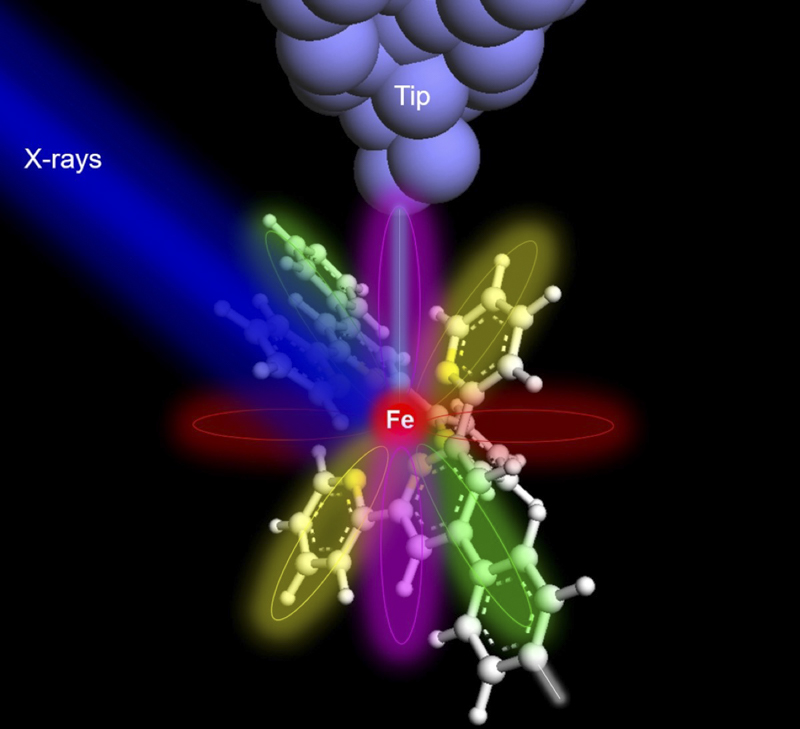 When X-rays (blue color) shine on an iron atom (red ball in the center of the molecule), the surface electrons of the nucleus are excited.
When X-rays (blue color) shine on an iron atom (red ball in the center of the molecule), the surface electrons of the nucleus are excited.